- Basic Search
- Chemical Similarity Search
- Chemical Property Search
MetaADEDB
MetaADEDB is an online database we developed to integrate comprehensive information of adverse drug events (ADEs).
Introduction of ADE
Adverse drug event (ADE) is defined as an injury during medication administration related to medicine, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product. ADEs lead to an enormous amount of morbidity and mortality. Previous studies showed that about 6% of patients were hospitalized due to ADE in the U.S., and ADEs caused 197,000 deaths in Europe per year. The more People usually take medicines as they age, the more the risk of adverse events may take place. The good news is the most of ADEs are preventable. So reducing adverse drug events is urgent to detect or determine ADEs and prevent the occurrence of ADEs.
Statistics of MetaADEDB
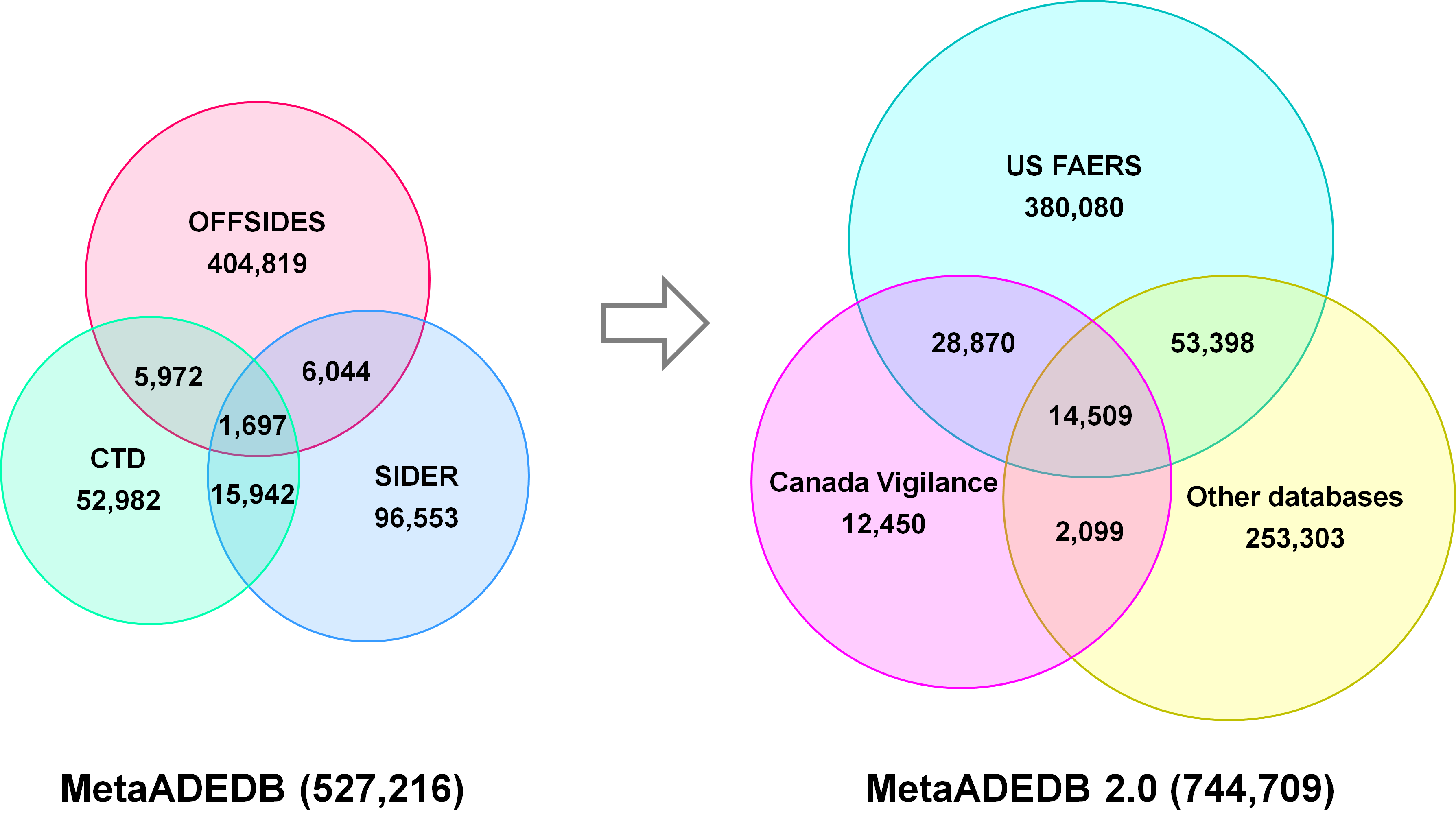
The new version consists of 744,709 drug-ADE associations between 8,498 compounds (including more than 3000 drugs) and 13,193 ADEs by integrating more and newer data from U.S. FDA Adverse Event Reporting System (FAERS) and Canada Vigilance Adverse Reaction Online Data-base in addition to the Comparative Toxicogenomics Database (CTD), SIDER and OFFSIDES. Additionally, Compared to the previous version, MetaADEDB 2.0 has an over 40% increase in drug-ADE associations and almost 200% growth in the total number of compounds. Notably, we directly generated a unique identifier for each compound based on the chemical structure, and UMLS ID was used as unique identifier for ADE to ensure the quality of data.
Tips
MetaADEDB is free for non-commercial use only. For any other use, please contact us.
Differences among three concepts
- "Adverse reaction" and "adverse effect" are interchangeable, except that an adverse effect is seen from the point of view of the drug, whereas an adverse reaction is seen from the point of view of the patient.
- "Adverse effect" and "adverse reaction" must be distinguished from "adverse event". An adverse effect is an adverse outcome that can be attributed to some action of a drug. An adverse event is an adverse outcome that occurs while a patient is taking a drug, but is not or not necessarily attributable to it.
News
- Novermeber 23, 2021, The download part was online.
- August 1, 2021, The paper of MetaADEDB was published by Bioinformatics.
- May 25, 2020, The MetaADEDB 2.0 was released.
- October 23, 2013, The paper of MetaADEDB was published by J. Chem. Inf. Model.
References
- Zhuohang Yu, Zengrui Wu*, Weihua Li, Guixia Liu, Yun Tang*. MetaADEDB 2.0: a comprehensive database on adverse drug events. Bioinformatics., 2021, 37 (15), 2221-2222.
- Feixiong Cheng, Weihua Li, Xichuan Wang, Yadi Zhou, Zengrui Wu, Jie Shen, Yun Tang*. Adverse Drug Events: Database Construction and in Silico Prediction. J. Chem. Inf. Model., 2013, 53 (4): 744-752.
Page last updated at 2020-05-25 10:01:57 (Asia/Shanghai) | You are visitor No. 9031
Copyright © 2019-2020 Laboratory of Molecular Modeling and Design, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology. All rights reserved.






